Case Report
A 24-year-old woman with an 8-year history of type 2 DM presented to the outpatient clinic of the orthopedic department with a complaint of left thigh pain since 1 month. She had been treated with metformin and multiple subcutaneous insulin injections (basal once-daily glargine plus multimeal aspart). The daily insulin requirement at the latest outpatient visit was 120 IU, but drug adherence was poor due to lack of her treatment willingness and familial support. She was also diagnosed as Prader-Willi syndrome at the time of her DM diagnosis based on her genetic testing that revealed deletion of chromosome 15q11-q13. She displayed signs of obesity, developmental delay, behavioral problems, and had a characteristic facial appearance. She also had a history of asthma, glaucoma, and low-grade anal squamous intraepithelial neoplasia. The possibility of deep vein thrombosis (DVT) was excluded based on the results of computed tomography (CT) angiography and venous Doppler ultrasonography of the lower extremities. Subsequently, she underwent magnetic resonance imaging (MRI) of her left thigh that showed enlargement and diffuse signal alterations in the adductor magnus muscle (
Fig. 1). Based on the preliminary MRI findings, the radiologist considered various diagnoses that included muscular sarcoidosis, proliferative myositis, or pyomyositis. The patient was referred to the rheumatologist and was admitted there for further examination.
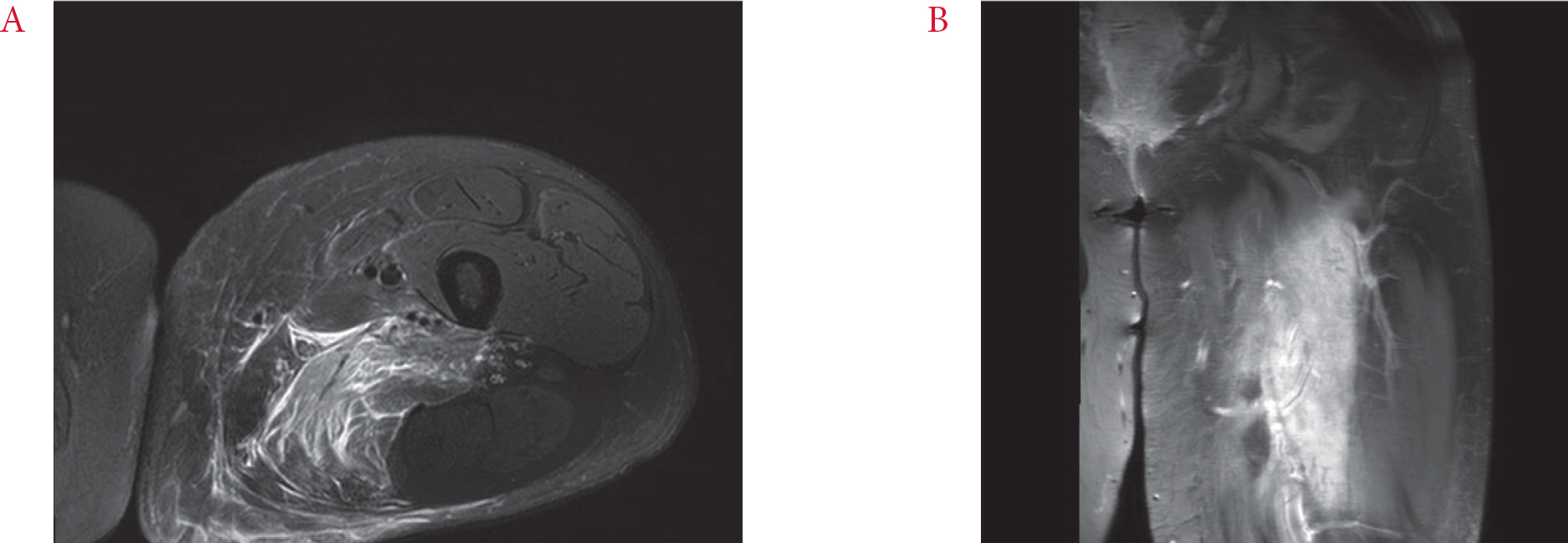
Fig. 1.
Magnetic resonance imaging of left thigh. (A) Axial T2-weighted image with fat suppression, demonstrating enlargement and diffusely increased signal intensity of the adductor magnus muscle and irregular hypointense area at the center. (B) Coronal T1-weighted, fat-suppressed image with gadolinium enhancement.
On admission, she was febrile (body temperature up to 38.7â) and showed a limping gait due to aggravated pain and swelling of the left thigh. On physical examination, compared with the right thigh, the anteromedial side of her left thigh was noticeably swollen (
Fig. 2) and extremely tender to palpation with a mild increase in local temperature. Laboratory studies revealed elevated creatinine kinase, 640 U/L (20 to 270 U/L); C-reactive protein (CRP), 13.9 mg/dL (< 0.3 mg/dL); and erythrocyte sedimentation rate, 104 mm/h (0 to 20 mm/h). However, other muscle enzymes such as lactate dehydrogenase and aldolase were within their normal ranges. Culture studies of blood, urine, and sputum specimens were negative. Additionally, at the time of admission, her elevated blood sugar level of 489 mg/dL and hemoglobin A1c (HbA1c) of 13.4% indicated poor glycemic control. Further, her blood samples were tested for the presence of autoantibodies and were found to be negative for rheumatoid factor, antinuclear antibody, anti-neutrophil cytoplasmic antibody, anti-cardiolipin antibodies, anti-ds DNA, anti-β2-glycoprotein I, anti-Ro, anti-La, anti Jo-1, and anti-RNP; however, they were positive for lupus anticoagulant. Chest CT findings were non-specific and not suggestive of sarcoidosis. Serum level of angiotensin-converting enzyme was within the normal range. For further evaluation, muscle biopsy was performed and skeletal muscle with dense interstitial infiltration of inflammatory cells (neutrophil-dominant), necrosis of muscle fibers, and adjacent panniculitis were observed on histopathology (
Fig. 3). Additional staining (Gram, Wright-Giemsa, Ziehl-Neelsen, Periodic acid Schiff, and Gomori methenamine-silver staining) of the biopsy sample yielded negative results that helped to rule out an infectious pathology. The polymerase chain reaction for
Mycobacterium tuberculosis complex and nontuberculous mycobacteria was also negative.
Fig. 2.
Diabetic muscle infarction involving the left thigh showing its enlargement compared with the right thigh.
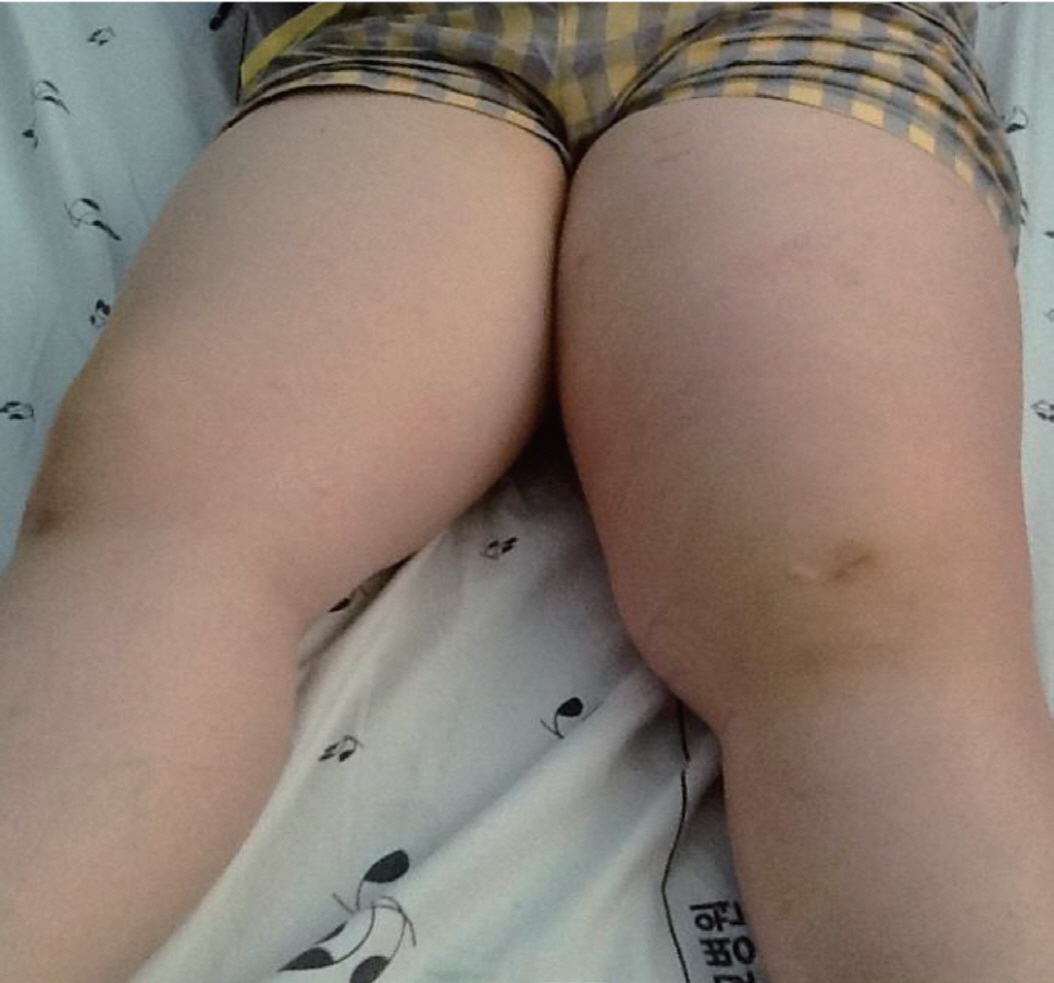
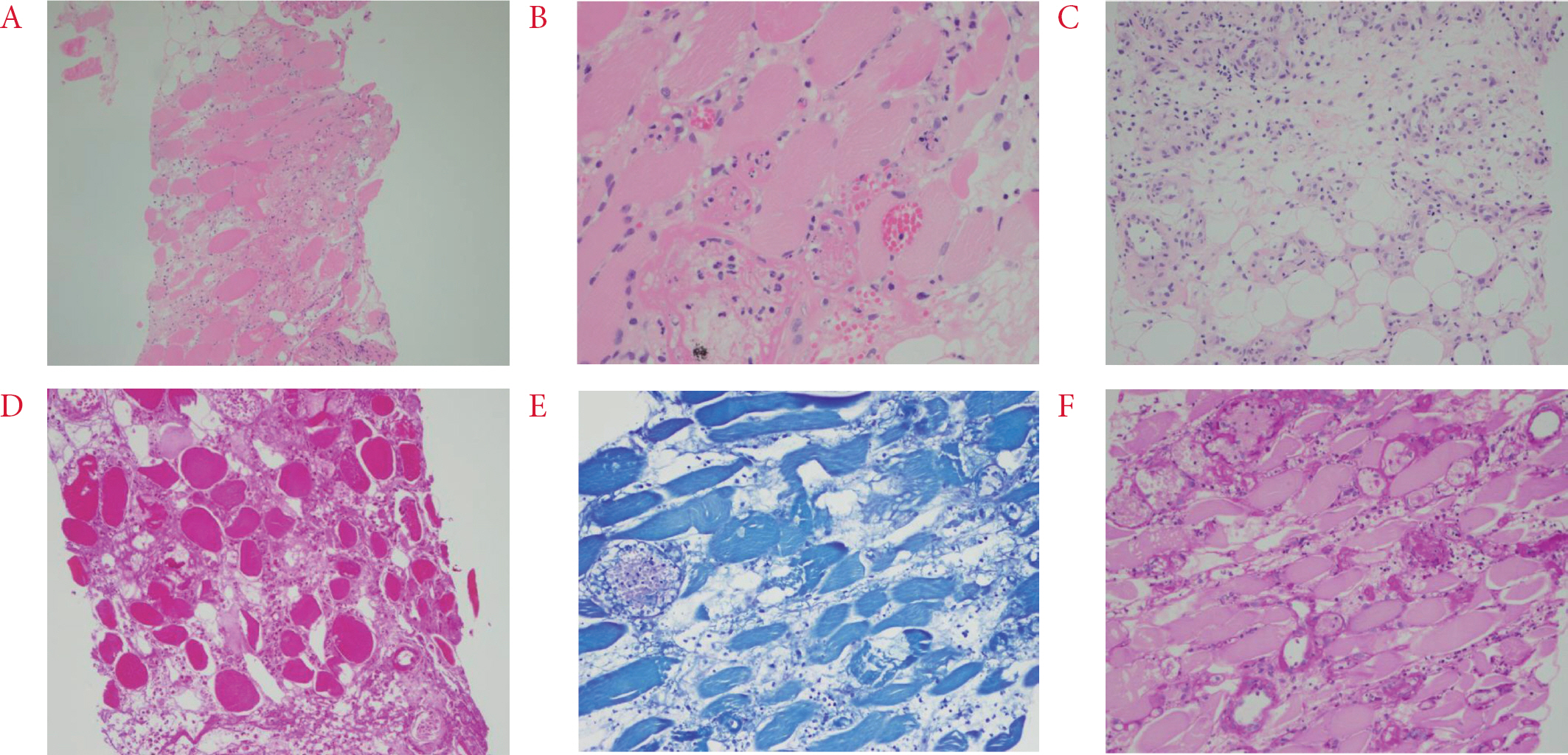
Fig. 3.
(AâźC) Biopsy of the left thigh skeletal muscle showing multifocal necrosis of muscle fibers and dens interstitial infiltration of neutrophils and lymphocytes, and panniculitis. H&E stain, Ă100; Ă200; Ă200, respectively. (DâźF) Gram stain, Wright-Giemsa stain, and Periodic acid Schiff stain for detecting bacteria and fungus were negative. Ă200.
The patient was initially treated with intravenous antibiotics, non-steroidal anti-inflammatory drugs (NSAIDs), and analgesics (oral opioids), because of sustained fever and the possibility of suppurative myositis on histopathology. To ensure strict glycemic control, the patient received multiple subcutaneous insulin injections with a daily insulin requirement of approximately 135 IU (basal glargine 60 IU plus premeal glulisine 25 IU) and three times of metformin 500 mg. Despite the antibiotic treatment, the CRP level was partially decreased and the swelling and tenderness of her left thigh did not improve. In consultation with an infection specialist, antibiotics were discontinued since her clinical course was not congruent with bacterial myositis. With this clinical information, MRI was reviewed again by a radiologist and the possibility of DMI was suggested. Even after discontinuing the antibiotics, her clinical symptoms and laboratory findings gradually improved with aggressive glycemic and pain control. Finally, DMI was diagnosed based on the exclusion of other causes and self-limitation of the disease progression with a conservative approach. Microvascular complications of DM were assessed by fundus examination that revealed mild, non-proliferative diabetic retinopathy in both eyes. She also had a neurogenic bladder, which is another possible neurologic complication of DM. Considering that four times of insulin injections were difficult to maintain for her treatment, we changed her insulin regimen to a premixed combination insulin injection twice a day before discharge. The level of HbA1c decreased to 7.0% after 3 months of discharge, and she is under follow-up for 6 months without any evidence of recurrence.
The study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB No. B-1910/568-702). The informed consent was obtained from the subject in accordance with the Declaration of Helsinki.
Discussion
We described a case of DMI in a 24-year-old woman with Prader-Willi syndrome and poorly controlled DM that was initially misdiagnosed as an inflammatory or infectious muscle disease. DMI is a rare complication that can occur in patients with longstanding DM and multiple microvascular complications. According to a recent systematic review of 126 patients with DMI, the mean age at presentation was 35.9 years and 52.2 years in type 1 and 2 DM, respectively, while the mean DM duration at the time of DMI diagnosis was 18.9 years and 11.0 years in type 1 and 2 DM, respectively [
2]. We did not suspect DMI at the first presentation, since our patient was relatively younger and had shorter disease duration compared with that of the previously reported cases.
Possible predisposing factors of DMI include longstanding and poorly controlled DM with coexisting complications that commonly include nephropathy, minimum of two microvascular complications, and triopathy in 75%, 66%, and 47% of the patients, respectively [
2]. At the time of presentation, our patient exhibited poor glycemic control, since she had been receiving insulin irregularly in the recent months due to poor insight and lack of familial support. Additionally, she suffered from two microvascular complications, including mild, non-proliferative diabetic retinopathy and an overactive bladder that could be attributed to diabetic neuropathy. The typical clinical symptoms of DMI are acute or subacute onset of muscular pain and swelling, especially involving the proximal lower extremities, without a history of trauma. Sometimes it accompanies a mass formation or fever, as was seen in our patient [
3]. Although non-specific, the laboratory results in DMI often show various abnormal findings, including an increase in leukocyte count, acute phase reactants, and muscle enzymes. These findings also suggest infectious or inflammatory muscle pathology and lead to a misdiagnosis. Negative culture studies can help to exclude the possibility of an infectious pathology.
In patients presenting with acute muscular pain and swelling, the differential diagnoses can include DVT, pyomyositis, necrotizing fasciitis, intramuscular hematoma, soft tissue abscess, ruptured Baker's cyst, and osteomyelitis [
4]. Doppler ultrasound or CT of the lower extremity can exclude DVT or abscess, while MRI is the choice of modality for diagnosing DMI. Typical MRI findings include diffusely hyperintense signal of affected muscle groups on T2-weighted, inversion-recovery, and gadolinium-enhanced sequences and isointense to hypointense signal on T1-weighted images, with associated perifascial, perimuscular, and/ or subcutaneous edema [
5,
6]. Further, intense diffuse FDG uptake has been reported in the affected muscles, in a DMI patient, who underwent FDG-positron emission tomography, to exclude a suspected infection [
7].
Muscle biopsy is not routinely recommended for the diagnosis of DMI due to the risk of procedure-associated complications and an associated increase in the duration of symptomatic improvement. However, for patients with atypical presentation, uncertain diagnosis, or inadequate treatment response, as in our case, muscle biopsy can provide useful clue for confirming the diagnosis. The gross findings of DMI show a non-hemorrhagic, pale, and whitish muscle. On light microscopy, DMI lesions demonstrate muscle necrosis and edema in the early stages and replacement of necrotic muscle fibers by fibrous tissue, neutrophilic and lymphocytic infiltration, and muscle regeneration in later stages [
2,
3].
Although the pathogenesis of DMI remains unclear, several hypotheses have been proposed, including ischemia-reperfusion injury, vasculopathic changes, and hypercoagulability. Early reports by Banker and Chester described embolization from an ulcerated atherosclerotic plaque in the abdominal aorta causing ischemia and muscle infarction [
1]. However, another report by the same authors that reviewed six cases of DMI, suggested the possibility of infarction caused by atherosclerotic occlusion of blood vessels of the thigh and secondary fascial compartment syndrome-like changes [
8]. Silberstein et al.[
9] suggested the theory of hypoxia-reperfusion injury in which the ischemic changes from diabetic microangiopathy and arteriosclerosis precipitate inflammatory response, hyperemia, and reperfusion with generation of reactive oxygen species, leading to muscle damage by direct and indirect effects of the compartment syndrome of the affected muscle. Conversely, Umpierrez et al.[
10] proposed vasculitis as the causative mechanism for DMI based on the muscle biopsy findings showing abundant perivascular inflammatory cell infiltration in some patients. They also suggested DMI to be a diabetic microvascular disease, since most DMI patients have multiple end-organ microvascular diabetic complications such as retinopathy, nephropathy, and peripheral neuropathy. Hypercoagulability and vascular endothelial damage may also contribute to the development of DMI. This is supported by several reports that have highlighted the coexistence of DMI and anti-phospholipid syndrome, increased rate of positivity for anti-cardiolipin antibodies in patients with type 1 DM, and acquired hypercoagulable states such as increased coagulation factors in DMI patients [
2,
11]. Our patient was positive for the lupus anticoagulant in accordance with the latter hypothesis.
Current treatments for DMI are mainly conservative. Rest, analgesia, and strict glycemic controls are primarily advised and the condition resolves over several weeks. The use of low-dose aspirin and NSAIDs in the acute period is recommended because of their anti-inflammatory and anti-thrombotic effects, unless contraindicated. Physiotherapy or surgical intervention is generally not recommended because of prolonged recovery time [
2]. Even after successful treatment, the recurrence rate of DMI is high, ranging from 34.9% to 43.9%, usually involving a different location/muscle group within 6 months [
2,
12]. Hence, careful follow-up after initial DMI management is required and further studies on strategies for preventing recurrence are needed.
To date, more than 10 cases of DMI have been reported in Korea [
13-
15]. Unlike the previous cases, the present case demonstrated the development of DMI in a relatively younger woman with shorter disease duration of type 2 DM. This may have been associated with her underlying diseaseâPrader-Willi syndromeâ and poor treatment adherence. Prader-Willi syndrome is associated with marked obesity and an increased risk for type 2 DM in adolescence [
16]. Although, there have been a few reports of diabetic complications in Prader-Willi syndrome, at present it is unclear whether DM-related complications are more frequent or severe in Prader-Willi syndrome patients [
16,
17]. Additionally, the association between thromboembolic event and Prader-Willi syndrome has been recently described [
18]. Although neither pulmonary thromboembolism nor DVT was detected on imaging studies, hypercoagulability of Prader-Willi syndrome itself might affect the susceptibility to DMI in our patient. To the best of our knowledge, this is the first description of DMI with supporting radiologic and pathologic findings in patients with Prader-Willi syndrome and type 2 DM. Additionally, as in our patient, when a patient presents with acute to subacute muscular pain and swelling with fever, it is difficult to diagnose DMI, preferentially, over inflammatory or infectious muscle conditions. It is further possible that physicians from the department of rheumatology, infectious diseases, or orthopedic surgery may encounter the patient first, before an endocrinologist. Finally, due to the rarity of DMI, a lack of awareness of the disease itself may lead to misdiagnosis and unnecessary administration of antibiotics or empirical steroids.
In conclusion, DMI is a rare complication of DM, commonly involving the lower extremities. Patients with genetic metabolic problems may develop DMI even at a young age. The possibility of DMI should be considered when patients with underlying poorly controlled DM present with acute to subacute pain, muscle swelling, and fever. Diagnosis could then be confirmed with imaging studies such as MRI and exclusion of other similar conditions.







